Every quarter the FDA (Food and Drug Administration) provides reports about adverse events, medication errors, and product quality complaints. The system used for that is the Federal Adverse Event Reporting System (FAERS) which is a database designed to support the FDA’s pharmacovigilance program for adverse events in response to drug and therapeutic biologic products and medication errors. Adverse events are associated with the use of medical products leading to unintended symptoms or diseases, often with harmful consequences.
We wanted to demonstrate a proof of concept application for accessing and analyzing FAERS data to test various hypotheses. Here we demonstrate the use of KNIME and FAERS to investigate the effects the medications Irinotecan and Loperamide have on Intestinal toxicity.
Irinocetan Medication for Cancer Treatment
Irinotecan has been used for the treatment of a number of solid tumors, including lung, colorectal, and pancreatic tumors. Its primary mode of action is via the inhibition of the topoisomerase I enzyme, which is responsible for DNA replication and transcription that causes cancer cells to survive and proliferate. as published in the paper, Individualization of Irinotecan Treatment: A Review of Pharmacokinetics, Pharmacodynamics, and Pharmacogenetics.
One of the serious dose-related toxicities is intestinal toxicity, in which a patient suffers from life-threatening conditions like diarrhea, depletion in fluid and electrolytes, and hospitalization .
Intestinal toxicity has two phases:
-
Early-onset diarrhea, which usually happens within 24 hours. This usually happens due to the cholinergic properties and is usually accompanied by other cholinergic symptoms like abdominal cramps.
-
Late-onset diarrhea occurs 24 hours after administration. The luminal irinotecan metabolites induce mucosal damage-causing malabsorption and imbalance with water and electrolytes.
Eventually, the gut-bacterial β-glucuronidase converts the irinotecan metabolite SN38G into an active form of SN38 that causes luminal damage and diarrhea, see Chemotherapy-induced diarrhea: pathophysiology, frequency and guideline-based management.
Thus a question we can ask is: Are there any medications, which, when administered with Irinotecan, have the potential to negate the onset of intestinal toxicity?
To answer this question, we tested the hypothesis that Irinotecan-driven intestinal toxicity is negated by the co-occurrence of Loperamide, a treatment used to treat diarrhea, which is discussed in the paper, New approaches to prevent intestinal toxicity of irinotecan-based regimens.
Our main hypothesis for testing is:
-
The number of Irinotecan-driven Intestinal toxicity cases is reduced with co-occurrence Loperamide
Which leads us to two sub-hypotheses:
-
The count of Loperamide use should be lower in those reporting intestinal toxicity and Irinotecan
-
The count of Loperamide use should be lower in those reporting Intestinal toxicity-related events and Irinotecan.
Hypothesis Testing on Adverse Events
First of all we downloaded the data from the FDA website. The data from every quarter needs to be downloaded separately and contains multiple files including demographic information, drug and reaction information from case reports and patient outcome information.
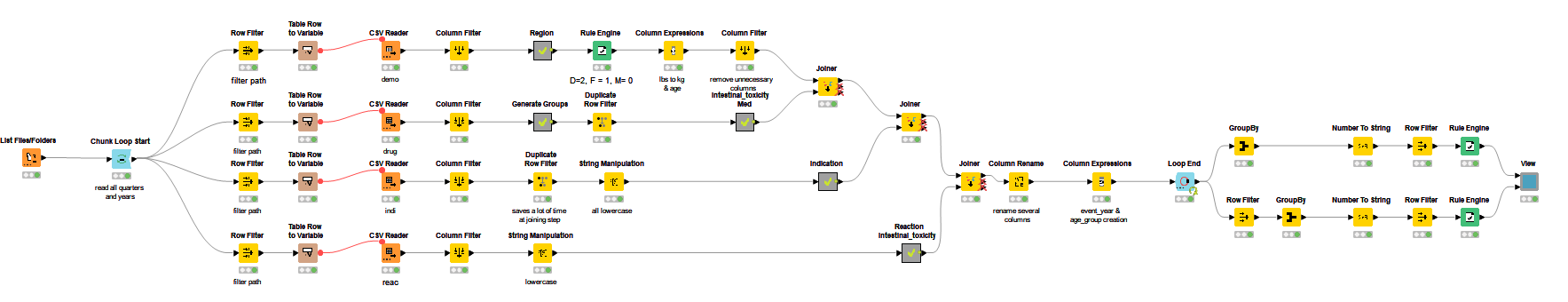
After reading in all the different CSV files, we grouped the patients by medication to get a general overview.
Group 1 includes all patients that took Irinotecan, Group 2 Irinotecan and Loperamide and Group 3 only Loperamide as medication.
We took data from 2015 through to Q3/2021. It indicated that simultaneous intake prescription of Irinotecan and Loperamide is not as common as taking both medicines by themselves.
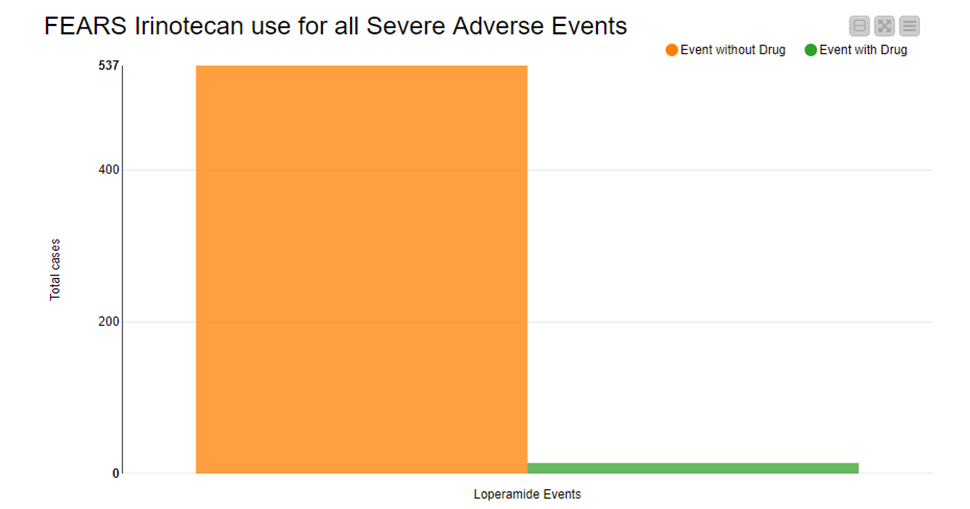
Afterwards we counted all the severe adverse events for both groups. As you can see in Figure 2, the patients who only took Irinotecan showed significantly more adverse events than those who took Irinotecan and Loperamide in combination.
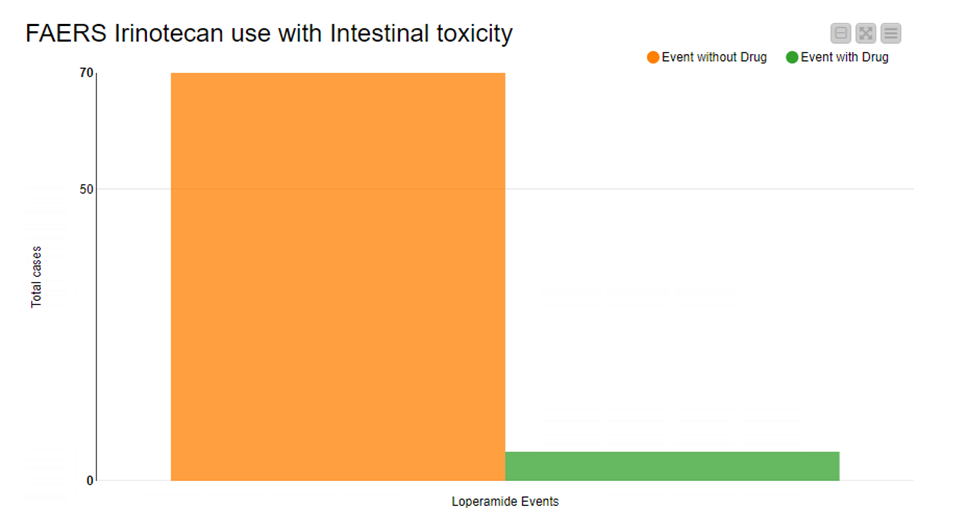
The analysis also shows us that more patients suffered from intestinal toxicity when using only Irinotecan as medication in comparison with patients who also took Loperamide in combination, seen in Figure 3.
Download and Query of FAERS Data Easily with KNIME
In summary, the workflow allows us to easily query the downloaded FAERS data for specific hypotheses testing. Download our publicly available workflows to use as your own starting point for processing FAERS data.
